Liquid metals, such as alloys of bismuth, gallium and indium,
potentially offer both low interfacial resistance and high conductivity.
Several alloys of gallium with very low melting points have also been
identified as potential liquid metal interface materials. Thermal performance
of such an interface would be more than one order of magnitude greater than
many adhesives typically in use.
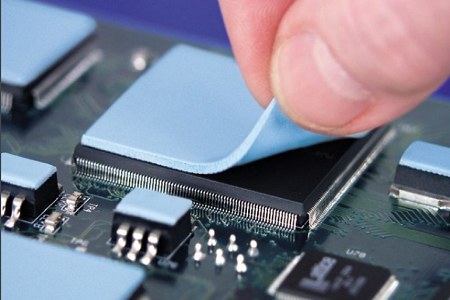
LMA alloys as a thermal interface material offer superior thermal
performance due to their high thermal conductivities and low contact
resistance, resulting from excellent surface wetting. Reworkability, ease of
handling, and a lack of cure make this attractive in a high volume setting. The
various failure mechanisms which have plagued the past and present LMA products
will be mitigated by applying a multidisciplinary approach to the challenge.
Liquid metals flow quite
well. The solid structures or phases proposed for incorporation within the
thermal interface address the basic problem of getting an LMA to stay put when
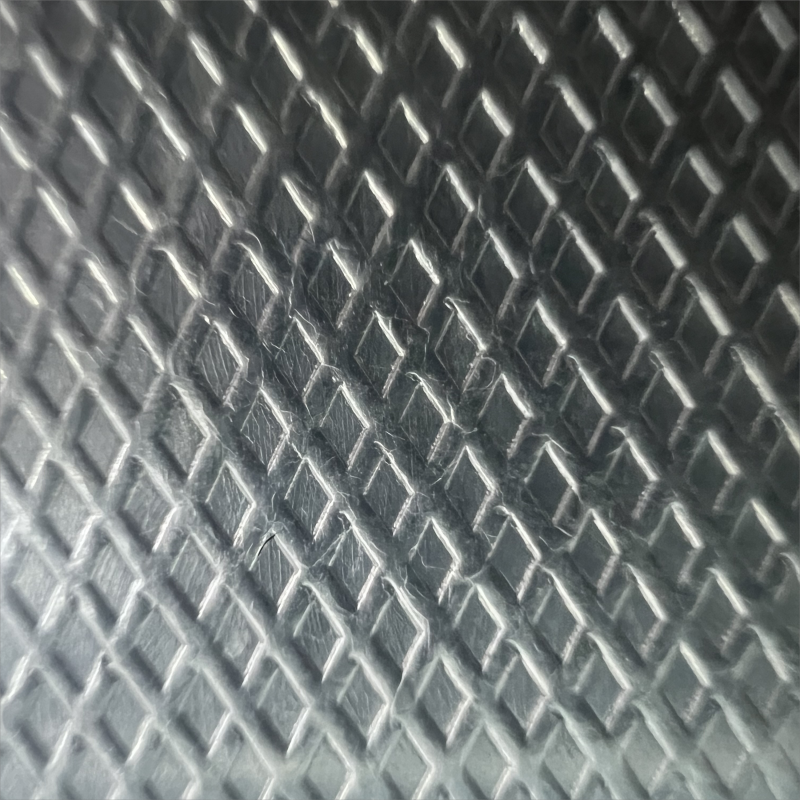
in service. These structures increase the surface contact area with the LMA in
the thermal interface. As long as the total solid-liquid interface energy is
less than the interface energy of the liquid-gas and solid-gas interfaces it
replaces, the LMA will minimize its surface energy by wetting the surfaces
within the interface. The LMA may still wet the surface adjacent to the thermal
interface if it is the same wettable surface used under the die, particularly
when acted upon by an additional force. Additional forces will arise from
shock, vibration, and CTE mismatches between the LMA and other components. Once
the LMA has wet the surface adjacent to, but outside the thermal interface, it
is doubtful surface tension alone will retain it within the interface when
acted upon by external forces. Others have proposed to address this difficulty
with gaskets to contain the LMA and fillers or non-eutectic (slushy)
compositions to increase its viscosity.
We have found that simply modifying the
surface around the thermal interface so that the LMA will not wet it is
sufficient to contain the LMA within the interface during shock, vibration, and
temp cycling. It is conceivable that if excess LMA were incorporated during
assembly, this excess could end up airborne from shock or vibration. Therefore,
LMA TIM should be deployed in closed cavities where no opportunities for shorts
or adverse reactions with other metals exist.